Models & Assays
PK & Safety
-
A Functional observation test battery for either the rat or mouse adapted from the Irwin (1968) test screen, and incorporating additional objective measures of motor and autonomic function.
-
Unique adverse effects may be suspected based on the pharmacological property or observations made during the drug characterization process. Additional concerns may arise during clinical trials. In such cases, follow-up studies are recommended (ICH S7A). We can provide the following supplemental services:
- Tests for dependence and abuse liability;
- Tests for cognitive liability; and
- Tests for pro-convulsant liability.
-
Animals can be chronically treated with an NCE under staggered designs such that 2nd/3rd level dosing can be determined by the effect at first dose. Daily observation, blood chemistry and cell counts are included. Additional options include: major organ histopathology; plasma sampling for toxicokinetic profiling; CSF sampling (in the dog); and incorporation of a functional CNS test battery into the study design. Our emphasis is on flexible designs to maximize the value of information to the customer to guide subsequent efficacy and GLP toxicology studies.
-
Frequently, drugs are administered in combination with another therapy. We offer the capability to test for potentially deleterious drug-drug interactions, both at the behavioural and pharmacokinetics level.
-
Animals can be treated with an NCE by either iv, sc, ip or oral routes of administration and plasma samples taken at multiple timepoints. Additional tissue samples, e.g. csf (rat) can be taken to determine CNS penetration of NCE. These can also be coordinated with efficacy studies to support PK-PD determinations.
-
FET evaluates the toxicity of compounds for zebrafish embryos and larvae. By exposing embryos to varying concentrations of a substance, we can monitor their development, survival, and any morphological abnormalities.
The introduction of 3,4-dichloroaniline culminated in numerous morphological alterations over a span of 96 hours. The most pronounced malformations included pericardial edema, yolk sac edema, as well as tail and head deformities as shown in the figure below.
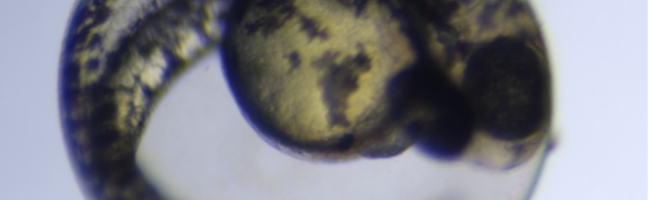
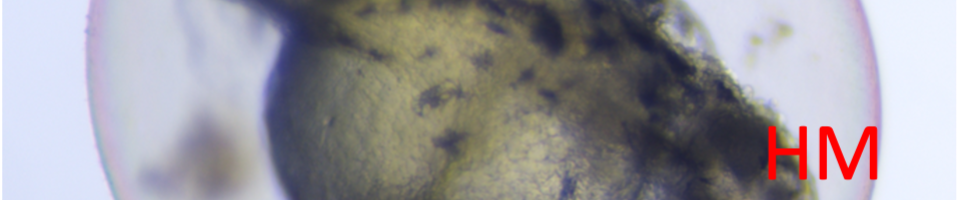
Figure 1. Embryo in normal physiological stage (left) at 48 hours post fertilization vs embryo after 4mg/l 3,4-dichloroaniline treatment (right) (PE – pericardial oedema; YSE – yolk sac oedema; TM – tail malformation; HM – head malformation)
CNS Drug Disposition
-
- Simultaneous collection of brain tissue and plasma from the same animal followed by LC-MS/MS bioanalysis of compound concentrations
- Brain and plasma free-fraction determination using equilibrium dialysis
- Useful for early-stage screening and lead selection programs, and can be combined with efficacy or POC studies
-
- Industry accepted gold-standard model for determining unbound drug concentrations in specific brain regions over time
- Combined drug and biomarker assessment enables PK-PD studies to establish robust dose-response profiles
- In vitro and in vivo parameters are optimized for each compound to ensure accuracy and reliability of results which can be used to support development decisions.
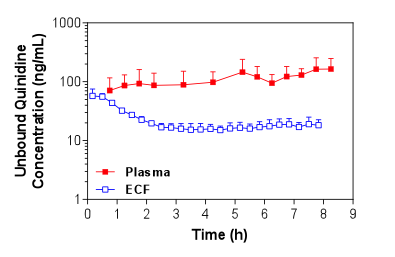 Figure 1. Mean + S.D. (n=4) unbound plasma and brain ECF concentrations of quinidine in Sprague-Dawley rats over time following i.v. administration of a loading dose and constant rate infusion of quinidine.
Figure 1. Mean + S.D. (n=4) unbound plasma and brain ECF concentrations of quinidine in Sprague-Dawley rats over time following i.v. administration of a loading dose and constant rate infusion of quinidine.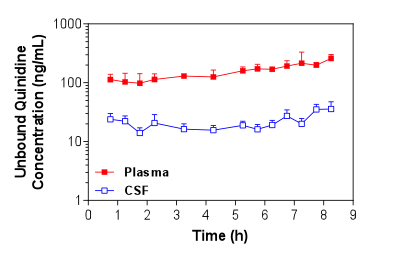 Figure 2. Mean + S.D. (n=5) unbound plasma and CSF concentrations of quinidine in Sprague-Dawley rats over time following i.v. administration of a loading dose and constant rate infusion of quinidine.
Figure 2. Mean + S.D. (n=5) unbound plasma and CSF concentrations of quinidine in Sprague-Dawley rats over time following i.v. administration of a loading dose and constant rate infusion of quinidine. -
- ~150 beagle dogs of various ages are available for CSF and plasma sampling studies to evaluate drug delivery and exposure in a clinically relevant non-rodent species
- Efficacy and safety profiles of new drugs can be further characterized by assessing drug or biomarker levels in CSF and plasma from the same animal over time
- Aged dogs provide a natural and translational model for various age related conditions such as Alzheimer’s Disease progression, Osteoarthritis and Cancer
-
- Collecting serial samples of CSF and plasma via surgically implanted catheters allows the determination of unbound drug delivery into the brain in awake rats
- Reduced inter-animal variability improves quality and power of data over parallel sampling techniques
- Studies can be performed in SAGE® ADME knockout rats to investigate how specific drug transporters affect the pharmacokinetics of drugs
- Data rich study designs have demonstrated predictive validity in characterizing drug delivery into the brain 1
- Fan, J. et. al. Drug Metabolism Reviews.44(S1):138-139 (2012)
-
- Non-survival sampling of cerebrospinal fluid from the cisterna magna of mice can be used to estimate free drug concentrations in the brain following drug administration
- Can be combined with brain tissue harvesting and plasma collection to understand differences in drug exposure for CSF, brain and plasma
- Also useful as an addition to efficacy or POC studies